
Verification of accuracy
There is only one sure way to verify that a sensor will respond to the gas for which it is designed: expose it to a known concentration of target gas and compare the reading with the concentration of the gas. This is referred to as a functional “bump†test.The safest course of action is to do a functional “bump†test prior to each day’s use. It is not necessary to make calibration adjustments unless readings are off by more than ten percent of the expected value.
But if your operating procedures do not permit daily sensor verification, it is possible to lengthen this interval. The International Safety Equipment Association (ISEA), working with a number of leading gas detection equipment manufacturers, has developed a protocol to clarify minimum conditions under which the interval between calibration checks may be lengthened, and has established a procedure to maintain prudent safeguards. Visit: http://www.safetyequipment.org/calibration.PDF.

Scale of calibration
Hot-bead combustible gas sensors are non-specific and respond to all combustible gases and vapors. It is not necessary for the combustible vapor to be present in LEL concentrations. Even trace amounts of combustible gas can be detected.A combustible gas sensor may be calibrated to any number of different gases. The gas used to calibrate the instrument is known as the “Calibration Standard.†If an instrument is only going to be used for a single type of gas, it should be calibrated to that gas. As long as the gas encountered is the same gas used during calibration, the readings will be exact (within the tolerances of the instrument design and the calibration gas used).
Figure 1 illustrates what may be seen when a combustible sensor is used to monitor gases other than the one to which it was calibrated. The chart shows the “relative response curves†of the instrument to several different gases. Note that the instrument response to the gas to which the instrument was calibrated (C) is accurate. For the other gases (A, B, D, E), however, the instrument responses are either higher or lower than the response to the gas to which it was calibrated.
Gases that produce higher readings than the calibration standard may result in the instrument going into alarm early. Gases that produce lower readings than the calibration standard can potentially result in a more dangerous error condition where the sensor essentially ignores threshold concentrations of combustible gas. One way to reduce the potential for this type of error is to use a lower alarm setting.
Another method for reducing the effects of low-reading error is in the choice of gas used to calibrate the combustible sensor. It may not be possible to calibrate directly to the gas measured, or the gas encountered may be unknown. In these cases, a mixture that provides an appropriate sensor response should be selected.
Relative response may be expressed as a ratio between the gas encountered and the calibration standard. Table 1 lists the expected response of a sensor that has been variously calibrated with pentane, propane, and methane to a variety of other combustible gases. The closer the relative response is to 1.0, the more accurate the reading.
When the instrument is calibrated to methane, readings for many gases on the list are dangerously low. On the other hand, when calibrated to pentane, readings for many gases are excessively high. When the instrument is calibrated to propane however, most of the gases on the list will produce readings that are quite close to actual. Thus, propane would seem to be the best choice. However, many users are more comfortable with the more “conservative†readings obtained from pentane calibration.
Still, neither of these calibration choices addresses the issue of sensor poisoning, and can lead to potentially dangerous consequences.

Sensor poisoning
Any hot-bead combustible sensor can be affected by the atmosphere in which it is used. Age and usage can also have a serious effect on sensitivity. Chronic exposure to substances containing silicone, the tetra-ethyl-lead found in “leaded†gasoline, halogenated hydrocarbons, high concentrations of hydrogen sulfide, and even exposure to very high concentrations of combustible gas may all lead to degraded combustible sensor performance.In most cases all this means is that the sensitivity is automatically adjusted upwards the next time the instrument is calibrated. In the worst case, the sensor may need to be replaced. Once again, verify the accuracy of the sensors on a regular basis.
But when a combustible gas sensor is poisoned by exposure to silicone, sensitivity tends to be lost first with regards to methane. This means the sensor may exhibit reduced sensitivity to methane while not exhibiting any loss of sensitivity to other gases. In extreme cases the sensor may not respond at all to methane while it still responds appropriately to other gases. It may even be possible to perform a calibration with these other gases (i.e., propane or pentane) and not notice the desensitization to methane. This condition can be very dangerous when the calibration gas used is based on any combustible gas other than methane.
This means that while methane does not typically provide an appropriate calibration scale, it is still important that the instrument be challenged with methane in order to recognize desensitized LEL sensors. But for many users it is not practical to obtain one calibration gas to establish a scale of calibration and then additionally use a methane gas to ensure that the LEL sensor has not been poisoned.
Using “Equivalent†calibration gases
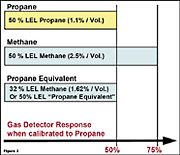
Figure 2 shows the relative response to propane and methane using a combustible gas sensor that has been calibrated to propane. When exposed to 50% LEL propane, the gas detector reading will be 50% LEL. When exposed to 50% LEL methane, the same detector will display a reading of approximately 75% LEL.If the concentration of methane is gradually reduced, the sensor response, and consequently the instrument readings, will be reduced as well. At some point the methane will be reduced to a concentration that will result in a display reading of 50% LEL. This level is 32% LEL methane or 1.62% methane by volume. In other words, if a combustible gas sensor that has been calibrated to propane is exposed to 32% LEL methane, the response will be equal to that of 50% LEL propane. This mixture of 1.62% methane by volume generates essentially the same sensor response as 50% LEL propane gas to all the gases listed in Table 1, and may therefore be considered “Propane Equivalent.†This relationship is shown in Figure 3.
The benefit of this Propane Equivalent is that a gas detector calibrated to 50% LEL propane equivalent gas will also detect (and provide additional adjustments for) desensitization should the sensor be poisoned by silicone. The same calculation can be done to establish a “Pentane Equivalent†calibration gas. In this case, the concentration needed to generate the same sensor response as 50% LEL pentane is 1.25% methane by volume.

