One of the most dangerous confined space entries is when a depleted oxygen, or inert, atmosphere is present. Inert, or “non-reactive,” atmospheres are used to displace oxygen or other reactive gases when the presence of that gas presents an explosion risk to either the process being performed or the compounds being stored. Examples of inert process applications include transportation or storage of flammable cargo, purged pressure vessels, catalytic processes in the petrochemical refining industry and welding operations.
These processes must maintain oxygen levels well below the combustion point for the catalyst or the chemicals being processed and therefore well below the viability point for any workers involved. All workers entering the space must be equipped with breathing apparatus, communications and gas detection. Because a traditional self-contained breathing apparatus (SCBA) does not provide a long enough work time and adds to the complexity of many tasks, the breathing apparatus of choice is a pressurized helmet driven by an external breathing air compressor. The helmet’s air supply must be monitored continually and workers often carry a small back-up breathing cylinder in case an emergency escape is required.
Nitrogen is the most commonly used inert gas, though others, such as “post-combustion” gases, are often used in transportation and storage applications to reduce the atmospheric presence of oxygen from its normal 20% to a range of less than 4% to 8%.
Catalytic cracking towers
Among the most often serviced inert confined spaces are catalytic cracking towers used in oil refineries to break down crude oil into different molecular weight compounds. Catalysts are substances that initiate or accelerate a chemical reaction without being consumed. Refinery hydro-treating reactors typically contain catalysts composed of the following elements: molybdenum (Mo), nickel (Ni), cobalt (Co). When the catalyst beds must be serviced, a technique called “gas blanketing” is used to prevent the external atmosphere from reacting with the catalyst and keeping it below the explosive range. Nitrogen is pumped into the vessel until it is purged, then a service technician with special life-support breathing helmet and protective suit enters the pressure vessel. Catalysts are removed from reactors in a highly reactive, sulfided state. The entire catalyst change-out process can take as long as a week to complete.
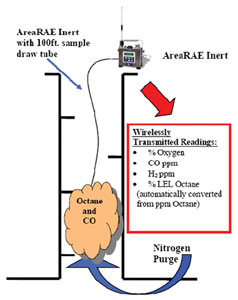
Technicians generally work with a safety-locked helmet with redundant air supplies, real-time radio contact with the safety team and personal backup controls. The operator of both the “purge atmosphere” and the worker breathing air works from a trailer on the ground. The purged atmosphere must be continually monitored for real-time oxygen level, explosive gases such as volatile organic compounds (VOCs) as well as carbon monoxide (CO).
Even though oxygen may have been purged from a space, it is still vital to accurately measure the concentration of both oxygen and explosive gases, since their toxicity remains unchanged and the danger of combustibility has been reduced, but not eliminated. A photoionization detector (PID) provides parts-per-million (ppm)-level detection of hydrocarbons for early warning of toxic and flammable gases.
Risks of working in an inert atmosphere
1. Inert atmosphere must be monitored for explosive gases, and any workers involved must have an adequate breathing supply.
2. Regular calibration and testing of the equipment used to generate inert gas is required to ensure that it works correctly. A sensor to measure the level of the inert gas and/or oxygen is needed to ensure atmosphere is not in the flammable range.
3. Inert atmosphere needs to be monitored for toxic gases. For example, in refinery catalyst maintenance, the presence of benzene, VCM, toluene, xylene, EDC, and other volatile organic hydrocarbons can exist.
Shortcomings of solutions used today
1. Traditional Wheatstone bridge/catalytic bead-based lower explosive limit (LEL) sensors require oxygen to detect flammable gases. A dilution fitting is used to introduce enough oxygen in the air to allow the LEL sensor to read properly. Dilution fittings are cumbersome and often used incorrectly.
2. LEL sensors only display in 1% increments. Most are not very accurate below 5% LEL. They cannot detect hydrocarbons at toxic levels (only flammable levels).
3. Remote monitoring by a second person is needed to increase the safety of workers in the inert atmosphere. Without a wireless system, users must hardwire traditional fixed LEL sensors to get remote readings.
Inert wireless monitoring system
1. A wireless monitoring system allows real-time remote monitoring of the inert atmosphere to ensure worker safety. Data can be shared by both the service provider and the site safety officer.
2. A wireless system is quick to deploy.
3. Sensitive photoionization detectors (PID) can be used to detect hydrocarbons resulting in readings in the parts-per-million range rather than percent.
4. A high-range carbon monoxide sensor can be used to measure the presence of CO, as well as hydrogen. This is in addition to the oxygen sensor which is used as a second sensor to ensure oxygen levels are low enough to prevent spontaneous explosion.
Measured results
• A specially calibrated PID is capable of accurate hydrocarbon or VOC measurements in zero- and low-oxygen environments.
• PID measurements of Hexane in an inert environment are typically within +/- 10% of the known value.
• PID measurements of Octane in a near-inert enivironment showed a 67% correlation with other instrument readings.
• Using the PID and correction factors to measure Octane and then converting to % LEL, the PID has a resolution of 0.02% LEL by volume when compared with existing systems that had a resolution of 3% LEL by volume.
