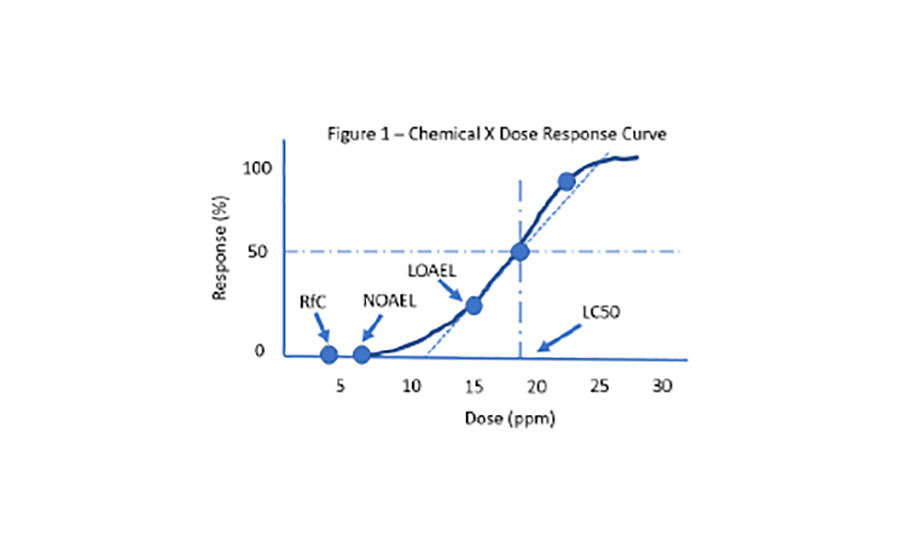
A basic understanding of the toxicological dose-response curve is a necessity for OHS pros. People fear most what they understand the least. New and vast toxicological information can trigger fear and irrational actions.
- LC50 is lethal concentration (inhalation) 50% kill of test animals.
- LC50 counterpart for oral or dermal dose is LD50 in mg/kg.
- LOAEL is the lowest observed adverse effect level.
- NOAEL is the no observed adverse effect level.
There is generally little controversy among toxicologists for LC50, LOAEL and NOAEL values.
Controversies
Controversy heats up for values below the NOAEL, where risk assessment comes into play.
RfC, for example, is an EPA term for reference concentration (there’s also RfD, too). RfC is the dose that humans may be repeated exposed during their lifetime without any significant harm.
The EPA has calculated an RfC for over 2,000 chemicals. A small number of RfCs make their way to regulatory limits.
The RfC is calculated by dividing the NOAEL or LOAEL by “uncertainty factors” (UF) in units usually from multiples 1-10. UF are necessary to extrapolate dose from animal to human, address exposure duration, and measure quality of data, among other things. The RfC calculation method is like how PELs and TLVs are created, with the exception that the UF for PELs and TLVs address working adults and not the general public.
The basic toxicological dose-response curve is universal, but once it gets within the purview of an agency such EPA, OSHA, ATSDR, or FDA, or organizations such as ACGIH, or is used outside the United States, different terminology occurs -- benchmark dose, threshold dose, acceptable daily intake, minimal risk levels, margin of error, etc. that often describes the same/similar concepts.
Figure 1 shows a linear slope dose-response curve. Controversy heats up when scientists interpret non-linear slopes for chemicals such endocrine disrupters or for carcinogens that don’t exhibit a threshold dose. These demonstrate U-shaped, J-shaped or bell-shaped dose-response curves.
Non-linear curves suggest that some chemicals may be harmful at low dose but safe at higher dosage. There may be no safe limit for some carcinogens. The best statistical model for the curve and label dose axis in log units (to better explain effects at extreme ends of the curve) are additional controversies. Another debate regards computational toxicology that may use chemical structure and physiochemical properties, rather than test animal dose, to predict adverse health effects.
DNELs
The dose-response curve is global. The Derived No-Effect Levels (DNELs), defined as the level of exposure above which humans should not be exposed, are required under EU REACH legislation. DNELs are calculated the same as the RfC, beginning with NOAEL or LOAEL values and then divided by “assessment factors.” Assessment factors are like the UF – different name for the same/similar concept.
The DMEL, Derived Minimal Effect Level, is required under REACH for carcinogens and other chemicals where no safe threshold can be derived. REACH requires a DNEL for each relevant exposure pattern including: systemic or local organ effects; acute and chronic exposure durations; oral, dermal or inhalation exposure routes; and, populations such as workers and the general public. One chemical, may have upwards of a dozen or more DNELs. Protection from adverse developmental effects such as an unborn child (GHS term) is an example of where different DNELs may be necessary for one chemical.
DNELs have revolutionized dose-response curve concepts. DNELs are developed by manufacturers or importers of chemicals into the EU. SECO DNEL (Simple European Calculator Of DNEL), available free, demystified the DNEL calculation process. May 2018 was the deadline (10-year timeframe) to register the lowest tonnage of dangerous substances under REACH.
Submitted registrations under REACH cover more than 20,000 substances now with more to come. Many of these substances have applicable DNELs. Contrast these thousands of substances against about 600 chemicals in the U.S. with either a worker PEL, TLV or REL; or a couple thousand chemicals with RfCs for public exposure within the EPA.
DNELs are readily available now for Internet public access and are becoming commonplace on U.S. employer-required HazCom Safety Data Sheets. DNELs are used for risk assessment purposes including, where applicable, worker exposure and product liability warnings. In the U.S., worker DNELs should be applied in absence of an applicable PEL, TLV or REL – although there is no present regulation that requires this action.
Worker/employer awareness
For nearly 50 years the dose-response curve was simple to explain to U.S. workers and employers. Below the LC50, LOAEL and NOAEL sat three primary worker exposure limits: the mandatory OSHA PEL, recommended ACGIH TLV, and to a much less degree, the NIOSH REL. These limits covered about 600 chemicals with generally one limit -- the most sensitive dose for each chemical.
The 2015 deadline for revised HazCom GHS, requiring exposure limits in section 8 of an SDS, presented new and differing exposure limits, such as various DNELs, to U.S. workers. The 2018 REACH deadline for registration of dangerous substances provides a vast, publicly accessible, toxicological database. The manufacturer/importer calculated DNEL provides worker exposure limits for thousands of chemicals that lack a PEL, TLV or REL.
The risk management concept that worker DNELs should be applied in absence of an applicable PEL, TLV or REL is prudent and ethically difficult to ignore.